Nicaragua Approves Emergency Use of Cuban Vaccines
Starting on October 20, Cuba’s vaccines against Covid-19 will arrive in Nicaragua to immunize children between 2 and 17 years of age.
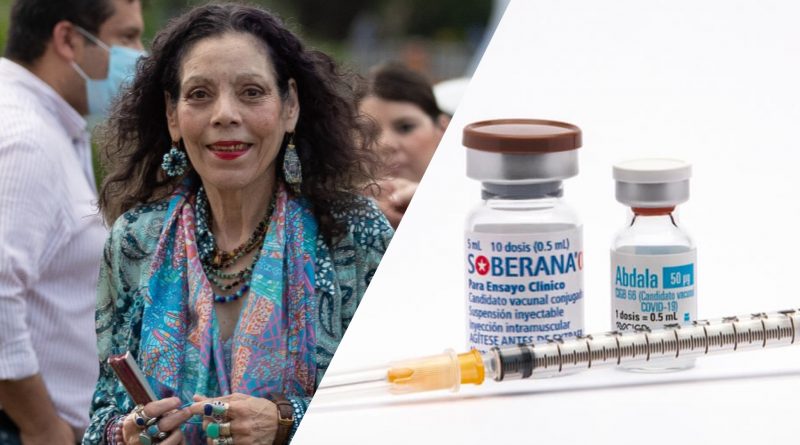
Abdala, Soberana and Soberana 2 have been approved for emergency use by the Ministry of Health and will be supplied by BioCubaFarma for the age group which is estimated at a little more than 2.3 million Nicaraguans. Minors may be vaccinated on a voluntary basis and only if their parents desire.
The announcement was made on Saturday afternoon by Vice President of Nicaragua, Rosario Murillo, through a message to Nicaraguans over national airwaves.
The certification granted by the Nicaraguan Regulatory Authority indicates that the Soberana vaccines and Abdala, are offered as a safe access therapeutic tool to reduce the transmissibility of Covid-19, produced by the Sars-Cov-2 virus.
With one of the highest vaccination rates in the Americas, Cuba intends to export the jabs to countries which would otherwise have difficulty obtaining vaccine supplies for their populations.
Vice President Murillo also indicated that vaccination is expected to soon expand to the remainder of the population above the age of 18, depending on the arrival of the vaccines through the Covax mechanism. 1,540,090 doses are scheduled to arrive in Nicaragua through the mechanism in the coming days.