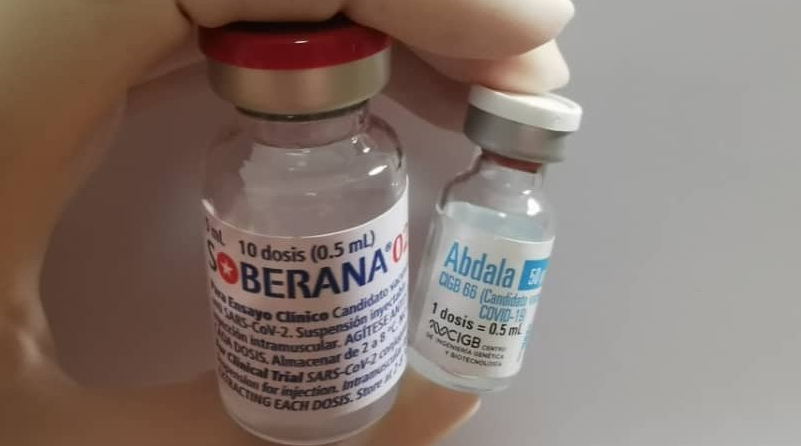
Two Cuban Vaccines Start Second Dose Phase III Trials
Thousands of volunteers in Cuba have started receiving the second doses of the Abdala and Soberana 02, as part of the third phase clinical trials of the country’s two COVID-19 vaccine candidates. If approved, it’ll be the first-ever Latin American COVID-19 vaccine.
The first Abdala doses began to be applied on March 22nd in the provinces of Santiago de Cuba, Guantanamo and Granma, and on April 3rd, authorities announced that the last of these doses had been administered to a total of 48,000 volunteers during a period of 10 days. The second dose must be received 14 days after the first.
Soberana 02 was the first Latin American vaccine to reach Phase III, the first doses were administered to 44,000 volunteers in Havana starting March 8th. The volunteers start receiving their second doses today, April 5th.
The World Health Organization (WHO) welcomed the news that the two Cuban vaccines showed positive results in the initial stages, and have managed to reach Phase III, the final phase before approval.
On January 22, WHO official José Moya said;
“I am optimistic about the results, they are going well. I had the opportunity to talk to the researchers, therefore I know that there is good progress on the Cuban vaccine candidates. We hope that this phase III concludes soon so that the Ministry of Health is able to make decisions regarding the vaccination program. The fact that Cuba is the first country in Latin America and the Caribbean to produce its own vaccine, is undoubtedly good news that makes all of us happy, especially that part of this production can also be made available to other countries in the region.”
According to the Cuban government, if the two vaccines pass all the trials, mass vaccination will begin in June, the government plans to have the entire island vaccinated by the end of 2021. There have been suggestions that tourists should be allowed to get the jab.
This extraordinary achievement is not surprising considering Cuba’s long history as a biotech powerhouse. The United States blockade on Cuba is aimed at isolating and starving the population by placing the country under siege. This has meant that many internationally produced medicines can’t reach the population due to obstacles placed by the U.S. government after the victory of the Cuban revolution. This has forced Cuba to become self-sufficient and produce its own medications and vaccines.
The Cuban model rejects corporate profiteering and instead focuses on state investment. The Center for Genetic Engineering and Biotechnology was the first such state institution, launched during Fidel Castro’s period in power. There’s now a huge network of different public institutes with 21 research centres in this field across the country. One of them is the Finlay Institute that produced Soberana 02. Thanks to this revolution in state-led Biotechnological research, Cuba now produces over 60% of the medications it uses domestically.
Cuba’s mission to become vaccine sovereign will help the island avoid the fate suffered by the rest of the region, that have found themselves at the mercy of foreign corporations and the unreasonable terms that are often demanded in exchange for jabs.
Argentinian government officials have revealed that Pfizer demanded sovereign assets, such as public buildings, as collateral in the case that citizens bring lawsuits for suffering adverse effects from its vaccine. If Cuba can vaccinate quickly upon approval, the revolution will have saved the population from looking abroad as the rest of Latin America has had to do.